A Silicon-Carbon battery is an advanced type of Lithium-ion battery that replaces or enhances the traditional graphite anode with a silicon-carbon composite. This technology offers up to 10 times higher theoretical energy density than pure graphite, allowing smartphones to have larger battery capacities (e.g., 6000 mAh) while remaining slim and lightweight.
Lately, a specific term has been creating a massive buzz in the world of mobile technology: “Silicon-Carbon (Si-C) Battery.” Industry experts and tech enthusiasts believe that this innovation is set to become a massive game-changer for modern smartphones.
But what is the reason behind this hype? Why is it being hailed as the new “Gold Standard” of mobile power?
To understand this, we need to dig deeper. The reality is that for a long time, the mobile industry has been desperately searching for a way to overcome certain deep-rooted battery limitations. As the saying goes, “Necessity is the mother of invention.” Whenever we face a persistent problem, a revolutionary solution eventually emerges.
The Daily Struggle: Why Current Batteries Are Failing Us
Before we dive into the technology, let’s look at the actual problems users face-challenges that led the industry to introduce the Silicon-Carbon battery as a definitive solution.
I am sure you encounter at least one or two of these frustrations yourself.
For instance:
You charge your phone to 100% in the morning, yet it struggles to last until the evening.
Your phone gets uncomfortably hot while charging. The device starts overheating during a long drive while using GPS.
Interestingly, battery struggles aren’t the same for everyone; they vary depending on where you are and how you live. Let’s look at three unique scenarios from different corners of the world to understand these pain points.
Three Global Corners, Three Unique Struggles

Scene 1: Sophie (A Fashion Influencer in Paris)
For Sophie, style is everything. She demands a phone that is sleek, elegant, and fits perfectly into her slim clutch bags. However, in the current tech world, a slim phone usually means a tiny battery. Sophie often finds herself forced to cut her high-engagement live streams short because her “beautiful” phone simply runs out of battery by late afternoon.
Scene 2: Marco (An Italian Adventurous Tourist in Ladakh)
Marco is standing by the breathtaking Pangong Lake in Ladakh. It’s evening, and the temperature has plummeted to a bone-chilling -5°C. Marco wants to capture a ‘Cinematic Video’ of this magical landscape. His phone shows 45% battery, which he assumes is plenty.
But the moment he hits the record button, the extreme cold causes the battery voltage to crash. In the blink of an eye, the battery drops from 45% to a mere 2%, and the phone flashes a ‘Battery Low’ warning. Marco is left baffled—where did all that power vanish without even being used?
Scene 3: Kabir (A Delivery Partner in Dubai)
In the scorching heat of Dubai, where the afternoon sun pushes temperatures past 50°C, Kabir is on the road for food deliveries. His phone is mounted on the bike’s handlebars, running GPS constantly with the screen brightness pushed to the maximum.
Kabir’s biggest fear isn’t the traffic; it’s his phone giving an “overheating” signal and shutting down. A dead phone means his work stops instantly. To make matters worse, he has to keep the phone plugged into a power bank, which makes the device even hotter.
The Common Link: The Lithium-Ion “Efficiency Wall”
These three stories represent very different problems in different parts of the world, but they all share a single culprit: The Traditional Lithium-Ion Battery.
Whether it is the lack of density for Sophie, the voltage drop in the cold for Marco, or the thermal instability for Kabir, the old technology has hit a wall.
As a solution to all these challenges, the industry has unveiled a breakthrough based on new-age science: The Silicon-Carbon (Si-C) Battery.
But how exactly does it solve these problems? Let’s move to the science behind the working of batteries.
Lithium-Ion vs. Silicon-Carbon:
Understanding the Core: Lithium-Ion vs. Silicon-Carbon
The main difference between Silicon-Carbon and Graphite batteries lies in their energy storage capacity. While traditional graphite has a limit of 372 mAh/g, silicon-carbon anodes can achieve practical capacities between 3000 and 3600 mAh/g. This means a Silicon-Carbon battery can store the same amount of power in nearly 1/10th of the weight and space required by a graphite battery.
To grasp why this is a revolution, we must first understand the fundamental differences between the traditional Lithium-ion battery we’ve used for years and the new Silicon-Carbon (Si-C) technology.
Let’s break this down in very simple terms.
Fundamentally, every battery consists of three main components:
- Cathode (The Positive terminal)
- Anode (The Negative terminal)
- Electrolyte (The Medium)
In a battery, there is a Cathode (+) plate on one side and an Anode (-) plate on the other.
During charging, ions flow from the Cathode to the Anode.
During discharging (usage), these ions flow back from the Anode to the Cathode. For these ions to move smoothly, they need a medium, and that is exactly what the Electrolyte does.
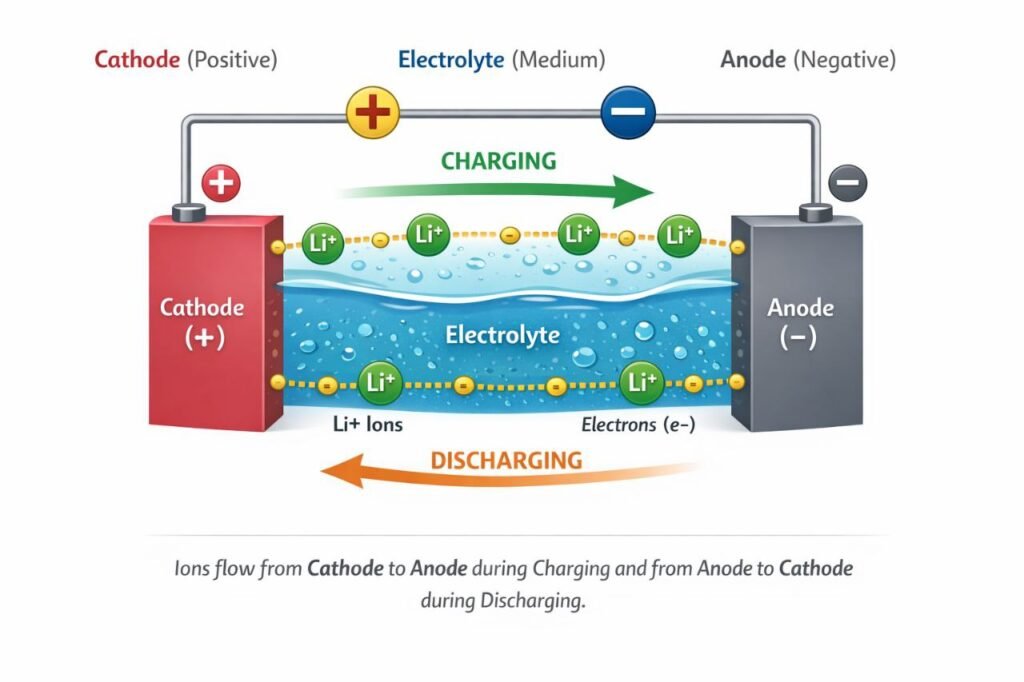
How does a lithium-ion battery work, and what are the problems associated with it?
In a standard battery, Lithium acts as the Cathode (+), while Graphite serves as the Anode (-). A lithium salt solution is used as the electrolyte.
Charging:
When you plug in your phone, Lithium-ions travel from the Cathode and settle into the Graphite.
Discharging:
When you unplug the phone and start using it, the ions begin to flow back from the Graphite (Anode) to the Lithium (Cathode). As this flow continues, your battery percentage gradually drops.
Draining: Why does the battery run out by evening?
Have you ever wondered why you charge your phone to 100% in the morning, yet it struggles to survive until sunset?
The reality is that our smartphone usage has exploded. From calling and messaging to video conferencing, YouTube, social media, and academic studies – we do everything on our phones.
Naturally, we need a high-power battery to keep up. However, the traditional Lithium battery is reaching its physical limits. It simply isn’t powerful enough to meet our modern expectations.
Our expectations are limitless, but its capabilities are very limited.
The Capacity Crisis: Why is the capacity of graphite so limited?
The reason lies in the molecular structure of graphite. It has a strict theoretical limit of 372 mAh/g. It simply cannot hold more energy than that, no matter how much we try to optimize it.
To understand this, let’s think of a parking lot.
If there are only 372 parking spaces, then no matter how many cars are lined up outside, only as many cars as there are spaces will fit inside.
The same is true for graphite. Lithium is constantly ready to send lithium ions, but the graphite can only accommodate as many ions as it has “space” for.
In contrast, silicon has many times more power. Let’s conduct a comparative study of these:
Capacity Comparison: Silicon vs. Graphite-Based Batteries

It is a well-known fact that 1 gram of graphite has a capacity of only 372 mAh (milliampere-hour).
To build a 5000 mAh battery, the theoretical requirement is:
Theoretical Weight Calculation
This calculation shows that a traditional graphite anode for a 5000 mAh battery would need to weigh at least 13.44 grams (of active material only).
However, in practical applications, 15 to 20 grams of graphite are actually used. Now, if we wanted to build a 10,000 mAh battery, we would need approximately 35 to 40 grams of graphite. In such a scenario, the smartphone would become extremely heavy and bulky.
In contrast, Silicon-based batteries require only 1.6 grams of silicon for a 5000 mAh capacity.
1 gram of silicon has the capacity to deliver 3000 mAh of current per hour. Although its capacity can be pushed up to 4300 mAh, it is currently maintained between 3000 and 3600 mAh for practical use.
For a 5000 mAh battery:
5000 / 3000 = 1.6 grams of Silicon.
Silicon-Carbon Advantage
Look at the huge difference! While graphite required 13.44g, silicon-carbon accomplishes the same task with just 1.6g. This is why new phones are so slim.
Even if we wish to build a 10,000 mAh battery, we would only need about 4 to 5 grams of silicon.
In short, Silicon’s capacity is many times higher than that of graphite. This allows manufacturers to build high-capacity batteries without increasing the weight of the phone.
| Feature | Graphite Anode | Silicon-Carbon Anode |
|---|---|---|
| Capacity per Gram | 372 mAh/g | 3000 – 3600 mAh/g |
| Weight for 5,000 mAh | 15 – 20 grams | ~1.6 grams |
| Weight for 10,000 mAh | 35 – 40 grams | ~4.5 grams |
| Physical Result | Heavy & Thick | Light & Slim |
Battery Capacity?
Battery capacity is represented in mAh (Milliampere-hours). You’ve likely seen ratings like 4500 mAh or 5000 mAh on your phone’s box. But what does a 5000 mAh battery truly signify?
Technical Deep Dive: What Does “5000 mAh” Actually Mean?
When we say a smartphone has a 5000 mAh battery, it doesn’t automatically mean the phone is more “powerful.” In technical terms, 5000 mAh (Milliampere-hour) represents the energy storage capacity.
It means the battery has enough stored energy to deliver a current of 5000 milliamperes for exactly 1 hour.
How long that battery lasts depends entirely on how quickly you “spend” that current. To put it simply:
- If you consume 5000 mA per hour, the battery will last 1 hour.
- If you consume 1000 mA per hour, it will last 5 hours.
- If you are a light user and consume only 500 mA per hour, it can last up to 10 hours.
The math is simple: the mAh rating tells you the total amount of current the battery can provide continuously for one hour. This is why tasks like high-end gaming or 4K video streaming drain the battery faster—they consume more milliamperes per hour.
Did you know?
It takes 6 Carbon atoms of Graphite to hold just 1 Lithium ion.
In contrast, 1 single Silicon atom can bond with up to 4 Lithium ions.
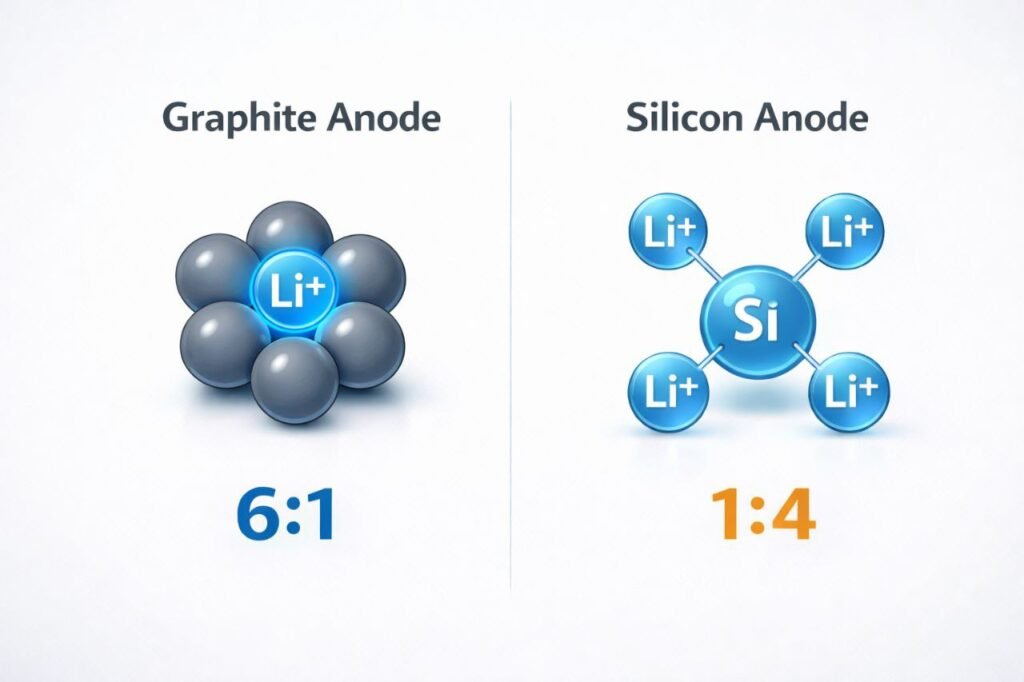
How do the new silicon-carbon batteries work, and why are they considered a game-changer?
It is important to understand that the Silicon-Carbon battery isn’t a completely new chemistry; it is still a Lithium-ion-based system.
The Setup:
The Cathode (+) remains Lithium. The Anode (-) still uses Graphite, but now, it is mixed with Silicon.
The Logic:
Because Graphite has a very low ion-absorption rate and Silicon has an incredibly high one, this mixture works perfectly to balance stability and power.
In short, the chemistry is the same, the system is familiar, but the storage capacity has skyrocketed.
Why does an Si-C battery charge faster and hold more power?
To understand this, let’s look at the “Teamwork” happening inside your phone during a charge.
What is the micro-science behind battery charging and discharging?
When you plug in your phone, Lithium-ions (Li+) travel from the Cathode through the electrolyte toward the Anode. But an ion alone cannot store energy; it needs a negative charge (-) to become a stable atom.
So when the ion (+) moves internally toward the Anode, an Electron (-) travels from the charger, through the phone’s external circuit, and meets the ion at the Anode. Together, they form a stable ‘Atom’ and are stored as chemical energy.
The Discharge (Usage):
The moment you start using your phone, the electrons rush back from the Anode to power your processor and screen.
Once the electron leaves, the Lithium-ion becomes “lonely” (positive again) and races back toward the Cathode to complete the cycle.
Thus, during charging, positive (+) ions and negative (-) electrons pair up, and during discharging, they separate. The positive ion then returns to the cathode. This cycle continues during both charging and discharging.
The Si-C Advantage:
Since graphite has limited space, its ability to form pairs of lithium (+) ions with (-) electrons is also very limited. In contrast, silicon can bond with many more ions simultaneously, allowing this “teamwork” to occur on a much larger and faster scale, which results in faster charging and longer-lasting battery life.
The Silicon Revolution: Why not earlier?
The advantage of Silicon-Carbon is clear: compared to old Graphite, Silicon’s structure manages the “union” of ions and electrons on a much larger scale and at a much faster pace. This is why phones are not only becoming slimmer but are also remaining super-fast and cool during charging.
In short, More capacity, faster charging.
However, a valid question arises: If Silicon was the answer all along, why wasn’t it used earlier?
The reason was a massive technical hurdle. When Silicon absorbs Lithium-ions, it physically expands—up to 300%. In the early stages of development, this expansion caused internal cracks in the battery, leading to a total performance failure within just a few months.
Carbon coating on silicon: Addressing the challenge of expansion

Modern Silicon-Carbon batteries have finally cracked this code. Scientists no longer leave Silicon “naked” or uncontrolled. Instead, they use three ingenious methods to keep it stable:
Carbon Coating (The Nano-Shield):
Silicon is now “wrapped” in Carbon. Microscopic particles of Silicon are encapsulated within a robust Carbon Shell.
Analogy: Think of placing a balloon inside a strong mesh cage. The balloon can expand, but the cage prevents it from stretching so far that it bursts.
Nano-sized Silicon Particles:
Instead of using large chunks, engineers now use nanometer-sized particles. Smaller particles mean less internal stress, minimal wear and tear, and a much longer battery life.
Flexible Binders:
In a battery, the material that holds everything together is called a ‘Binder.’ Modern Si-C batteries use advanced binders that act like rubber—they are flexible enough to absorb the expansion and contraction without breaking the circuit.
The Verdict: Power Without the Bulk
The problem that plagued Silicon technology for years has finally been solved. This breakthrough allows smartphone companies to make a bold claim: “We can increase battery capacity without increasing the battery’s physical size.”
The conclusion is straightforward
In the old Lithium-ion era, the limit was set by Graphite, which was very low—approx. 372 mAh/g.
In the new Silicon-Carbon era, that limit has jumped to nearly 3000 mAh/g.
In other words, the same parking lot that could previously accommodate only 372 cars has now become a multi-storey parking facility—without increasing the land area, it can now accommodate many times more cars.
By using Si-C technology, we can either make phones that last significantly longer or reduce the battery’s footprint to make room for other innovations—like larger Camera Sensors for better photography.
For the average user, this advanced tech translates to one simple promise:
“Your phone will now last longer, without becoming heavier or bulkier.” 😊
Real-World Solutions: How Si-C Technology Fixes Daily Struggles
Now, let’s see how the Silicon-Carbon battery provides a definitive solution to the diverse problems faced by our three global users.
Scene 1: For Sophie: Power Without the Bulk
Sophie wants a slim phone that fits effortlessly into her clutch bags. Previously, a slim design meant compromising on battery life. Now, mobile manufacturers can create incredibly thin phones without sacrificing power. Thanks to Si-C technology, people like Sophie can enjoy “pocket-friendly” aesthetics with “pro-level” battery backup.
Scene 2: Marco’s Struggle (The Cold Weather Mystery)
Why do batteries crash in the cold? The reason is electrochemistry. In freezing temperatures, Lithium-ions get “trapped” or sluggish within the rigid structure of Graphite, causing the flow of electricity to stall.
The cold turns the electrolyte into a ‘traffic jam’ for ions, but Si-C provides an ‘express lane’.
However, Silicon-Carbon batteries feature a unique Carbon Matrix structure that prevents ions from freezing up. This allows the battery to function with the same agility at -20°C as it does at room temperature. For travelers like Marco, this means the phone won’t give up in the middle of a snowy landscape.
Scene 3: Kabir’s Challenge (Beating the Heat)
Many users complain about their phones overheating during charging or while using GPS during a long drive. Let’s see how Silicon-Carbon technology tackles this.
Are phones with Si-C batteries free from overheating issues?
Yes, phones with Si-C batteries do not experience overheating problems.
This is because silicon-carbon batteries are safer and generate less heat compared to traditional lithium-ion batteries.
Silicon-Carbon (Si-C) batteries reduce overheating through lower internal resistance and superior thermal insulation. Unlike traditional graphite batteries, where ions struggle to move and create “friction heat,” the Si-C structure allows a much smoother flow of lithium ions.
Additionally, the silicon particles are encapsulated in a ‘Carbon Nano-Shield’ that acts as a heat insulator, protecting the battery from external temperatures and preventing thermal shutdowns during heavy tasks like gaming or fast charging.
Thanks to two major advancements:
Lower Internal Resistance:
In traditional batteries, ions struggle to move through graphite, creating internal “friction” and heat. The Si-C structure allows a much smoother flow. This reduces heat generation, keeping the phone cool even during heavy tasks like 4K recording or long-distance navigation.
Thermal Insulation (The Nano-Shield):
Silicon particles are encapsulated in a ‘Carbon Shell’ that acts as a heat insulator. This shield prevents external heat (like Dubai’s 50°C sun) from reaching the sensitive internal chemicals, effectively preventing ‘Overheating Shutdowns’.
The Verdict:
Your phone stays cool because the battery generates less heat internally and resists external heat better.
Longevity and Fast Charging: Designed to Last
In an emergency, just 15-20 minutes of charging can now provide hours of backup. But this brings up a common concern: Does fast charging kill the battery?
The answer is – NO.
However, there is a common misconception that “more power = faster degradation.”
But Si-C batteries are designed for ultra-fast charging without generating excessive heat.
Traditional Lithium-ion (Graphite) batteries did indeed have a shorter lifespan—usually dying after 1.5 to 2 years of heavy use.
But Silicon-Carbon changes the math. This technology is optimized for speed:
High Ion Absorption: Graphite causes ions to “crowd up” at the entrance, generating heat. Silicon, however, absorbs ions almost instantly.
Durability: Because it absorbs ions so efficiently, the battery can handle 100W or 120W super-fast charging without any cell damage.
While old batteries would start losing their health after 500 charge cycles, Si-C batteries maintain peak performance for 1000+ cycles.
In simple terms, your battery life is now doubled—lasting comfortably for 3 to 4 years instead of just two.
Double the Lifespan: How Si-C Batteries Last 4+ Years
The lifespan of a Silicon-Carbon battery is approximately double that of a traditional one. While a standard battery is rated for around 500+ charge cycles, Si-C batteries can easily handle 1000+ cycles.
In simpler terms, a traditional battery starts to weaken and lose its health after 1.5 to 2 years (around 500 charges). However, Silicon-Carbon technology maintains peak performance for 3 to 4 years (1000+ charges). This means your phone stays “as good as new” for a much longer period.
New Technology & The Big Challenge: Why isn’t Silicon-Carbon in every phone?
Despite its revolutionary advantages, this technology has not yet reached every consumer. There are two primary reasons for this:
Budget & Mid-range Phones (The Cost Factor):
This technology is currently quite expensive for affordable smartphones. While traditional graphite is abundant and cheap, the nano-processing required to make Silicon battery-ready significantly increases production costs.
In the budget segment, even a minor additional cost impacts the final price, making it challenging for the phone to remain competitive against its rivals.
Premium Brands like Apple (Trust & Supply Chain):
Premium brands meticulously evaluate every aspect of a new technology—such as its reliability, necessity, cost-efficiency, and global supply chain—before adoption.
Specifically, in the case of Apple, it is a well-known fact that they never rush to be the first to adopt a new technology. Apple’s strategy is ‘Reliability First.’
They wait until the technology is fully mature and its global supply chain is stable enough to cater to the demand of millions of iPhones.
Why is this technology still limited?
Currently, this technology is expensive. For affordable smartphones, graphite anode remains the cheapest and most durable option.
Apple does not rush to adopt new technology. They wait until the supply chain and long-term safety are 100% guaranteed.
The Global Market: Is This the Future of Your Next Phone?
The real-world needs of millions of users like Marco, Sophie, and Kabir have pushed tech giants to evolve. As a result, major global brands have already made Si-C technology a standard in their flagship models.
Soon, this will trickle down to mid-range and budget segments as well.
However, while you wait for this technology to reach your next device, it is equally important to know how to choose the best phone available right now.
To make a smart and informed decision, don’t forget to check out our comprehensive [Smartphone Buying Guide].
Frequently Asked Questions (FAQs)
Q1. Does it need a special charger?
Ans. No, it works with standard Type-C chargers, but original high-wattage chargers give the best results.
Q2. Is it safe?
Ans. Yes, it is safer than old batteries due to its controlled nano-structure and flexible binders.
Q3. How to identify a Si-C battery?
Ans. Look for “Silicon-Carbon Anode” in specs. If a phone is very slim but has a 6000 mAh battery, it’s Si-C tech.
